Biblio du mois : Mai 2019
En Mai, fais ce qu’il te plait !
Et ce qui vous plait, c’est la biblio du mois de l’AJAR Paris bien sûr 😉
Un programme chargé pour une petite mise à jour avec des recommendations GIHP sur la gestion des anti-agrégants plaquettaires avec une mention spéciale pour le Ticagrelor où une étude le placerait comme un nouvel antibiotique et un antidote possible qui arriverait sur le marché.
Sinon de la Neuroréanimation comme on aime avec discussion sur l’hypothermie (qui en fait encore ?), thrombolyse d’AVC no limit ? Du Delirium et même d’individualisation des objectifs de PAM en fonction du seuil d’autorégulation cérébrale en chirurgie cardiaque ! Recommendations péri-opératoire de chirurgie cardiaque.
On citera de beaux articles sur le SDRA (débat curares), l’application de la driving pressure en chirurgie thoracique jusqu’à de l’ALR en tout genre avec débat canal des adducteurs pour les PTG !
Si l’intelligence artificielle vous intéresse, un nouvel article définissant 4 profils de sepsis. Merci le Machine Learning…
Alerte dogme faux sur la prévention de TVP post-op par Lovenox 4000 UI SC à tous…
Quand on dit que notre spécialité est transversale !
On vous rappelle que pour en profiter au maximum, on vous a fait un tuto : ici
Et n’oubliez pas si vous vous ennuyez en ce début de semestre qu’on vous avez repris un Top 100 des articles indispensables en Réa : https://www.ajar-online.fr/top-100-des-articles-indispensables-de-reanimation/
Sédation précoce par Dexmedetomidine en Réa : NS ?
Yahya Shehabi, et al., NEJM, 2019
DOI: 10.1056/NEJMoa1904710
https://www.nejm.org/doi/10.1056/NEJMoa1904710
Background
Dexmedetomidine produces sedation while maintaining a degree of arousability and may reduce the duration of mechanical ventilation and delirium among patients in the intensive care unit (ICU). The use of dexmedetomidine as the sole or primary sedative agent in patients undergoing mechanical ventilation has not been extensively studied.
Methods
In an open-label, randomized trial, we enrolled critically ill adults who had been undergoing ventilation for less than 12 hours in the ICU and were expected to continue to receive ventilatory support for longer than the next calendar day to receive dexmedetomidine as the sole or primary sedative or to receive usual care (propofol, midazolam, or other sedatives). The target range of sedation-scores on the Richmond Agitation and Sedation Scale (which is scored from −5 [unresponsive] to +4 [combative]) was −2 to +1 (lightly sedated to restless). The primary outcome was the rate of death from any cause at 90 days.
Results
We enrolled 4000 patients at a median interval of 4.6 hours between eligibility and randomization. In a modified intention-to-treat analysis involving 3904 patients, the primary outcome event occurred in 566 of 1948 (29.1%) in the dexmedetomidine group and in 569 of 1956 (29.1%) in the usual-care group (adjusted risk difference, 0.0 percentage points; 95% confidence interval, −2.9 to 2.8). An ancillary finding was that to achieve the prescribed level of sedation, patients in the dexmedetomidine group received supplemental propofol (64% of patients), midazolam (3%), or both (7%) during the first 2 days after randomization; in the usual-care group, these drugs were administered as primary sedatives in 60%, 12%, and 20% of the patients, respectively. Bradycardia and hypotension were more common in the dexmedetomidine group.
Conclusions
Among patients undergoing mechanical ventilation in the ICU, those who received early dexmedetomidine for sedation had a rate of death at 90 days similar to that in the usual-care group and required supplemental sedatives to achieve the prescribed level of sedation. More adverse events were reported in the dexmedetomidine group than in the usual-care group.
Curarisation précoce dans les SDRA modérés à sévères : NS ?
The National Heart, Lung, and Blood Institute PETAL Clinical Trials Network
https://www.nejm.org/doi/10.1056/NEJMoa1901686
DOI: 10.1056/NEJMoa1901686
Background
The benefits of early continuous neuromuscular blockade in patients with acute respiratory distress syndrome (ARDS) who are receiving mechanical ventilation remain unclear.
Methods
We randomly assigned patients with moderate-to-severe ARDS (defined by a ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen of <150 mm Hg with a positive end-expiratory pressure [PEEP] of ≥8 cm of water) to a 48-hour continuous infusion of cisatracurium with concomitant deep sedation (intervention group) or to a usual-care approach without routine neuromuscular blockade and with lighter sedation targets (control group). The same mechanical-ventilation strategies were used in both groups, including a strategy involving a high PEEP. The primary end point was in-hospital death from any cause at 90 days.
Results
The trial was stopped at the second interim analysis for futility. We enrolled 1006 patients early after the onset of moderate-to-severe ARDS (median, 7.6 hours after onset). During the first 48 hours after randomization, 488 of the 501 patients (97.4%) in the intervention group started a continuous infusion of cisatracurium (median duration of infusion, 47.8 hours; median dose, 1807 mg), and 86 of the 505 patients (17.0%) in the control group received a neuromuscular blocking agent (median dose, 38 mg). At 90 days, 213 patients (42.5%) in the intervention group and 216 (42.8%) in the control group had died before hospital discharge (between-group difference, −0.3 percentage points; 95% confidence interval, −6.4 to 5.9; P=0.93). While in the hospital, patients in the intervention group were less physically active and had more adverse cardiovascular events than patients in the control group. There were no consistent between-group differences in end points assessed at 3, 6, and 12 months.
Conclusions
Among patients with moderate-to-severe ARDS who were treated with a strategy involving a high PEEP, there was no significant difference in mortality at 90 days between patients who received an early and continuous cisatracurium infusion and those who were treated with a usual-care approach with lighter sedation targets.
En vidéo : https://www.nejm.org/do/10.1056/NEJMdo005521/full/
Thrombolyse jusqu’à 9h pour les AVC ischémiques guidée par l’IRM !
Henry Ma, et al., N Engl J Med 2019; 380:1795-1803
https://www.nejm.org/doi/10.1056/NEJMoa1813046
DOI: 10.1056/NEJMoa1813046
Background
The time to initiate intravenous thrombolysis for acute ischemic stroke is generally limited to within 4.5 hours after the onset of symptoms. Some trials have suggested that the treatment window may be extended in patients who are shown to have ischemic but not yet infarcted brain tissue on imaging.
Methods
We conducted a multicenter, randomized, placebo-controlled trial involving patients with ischemic stroke who had hypoperfused but salvageable regions of brain detected on automated perfusion imaging. The patients were randomly assigned to receive intravenous alteplase or placebo between 4.5 and 9.0 hours after the onset of stroke or on awakening with stroke (if within 9 hours from the midpoint of sleep). The primary outcome was a score of 0 or 1 on the modified Rankin scale, on which scores range from 0 (no symptoms) to 6 (death), at 90 days. The risk ratio for the primary outcome was adjusted for age and clinical severity at baseline.
Results
After 225 of the planned 310 patients had been enrolled, the trial was terminated because of a loss of equipoise after the publication of positive results from a previous trial. A total of 113 patients were randomly assigned to the alteplase group and 112 to the placebo group. The primary outcome occurred in 40 patients (35.4%) in the alteplase group and in 33 patients (29.5%) in the placebo group (adjusted risk ratio, 1.44; 95% confidence interval [CI], 1.01 to 2.06; P=0.04). Symptomatic intracerebral hemorrhage occurred in 7 patients (6.2%) in the alteplase group and in 1 patient (0.9%) in the placebo group (adjusted risk ratio, 7.22; 95% CI, 0.97 to 53.5; P=0.05). A secondary ordinal analysis of the distribution of scores on the modified Rankin scale did not show a significant between-group difference in functional improvement at 90 days.
Conclusions
Among the patients in this trial who had ischemic stroke and salvageable brain tissue, the use of alteplase between 4.5 and 9.0 hours after stroke onset or at the time the patient awoke with stroke symptoms resulted in a higher percentage of patients with no or minor neurologic deficits than the use of placebo. There were more cases of symptomatic cerebral hemorrhage in the alteplase group than in the placebo group.
Antidote du Ticagrelor en cours ?
Deepak L. Bhatt, et al., N Engl J Med 2019; 380:1825-1833
https://www.nejm.org/doi/10.1056/NEJMoa1901778
DOI: 10.1056/NEJMoa1901778
Background
Ticagrelor is an oral P2Y12 inhibitor that is used with aspirin to reduce the risk of ischemic events among patients with acute coronary syndromes or previous myocardial infarction. Spontaneous major bleeding and bleeding associated with urgent invasive procedures are concerns with ticagrelor, as with other antiplatelet drugs. The antiplatelet effects of ticagrelor cannot be reversed with platelet transfusion. A rapid-acting reversal agent would be useful.
Methods
In this randomized, double-blind, placebo-controlled, phase 1 trial, we evaluated intravenous PB2452, a monoclonal antibody fragment that binds ticagrelor with high affinity, as a ticagrelor reversal agent. We assessed platelet function in healthy volunteers before and after 48 hours of ticagrelor pretreatment and again after the administration of PB2452 or placebo. Platelet function was assessed with the use of light transmission aggregometry, a point-of-care P2Y12 platelet-reactivity test, and a vasodilator-stimulated phosphoprotein assay.
Results
Of the 64 volunteers who underwent randomization, 48 were assigned to receive PB2452 and 16 to receive placebo. After 48 hours of ticagrelor pretreatment, platelet aggregation was suppressed by approximately 80%. PB2452 administered as an initial intravenous bolus followed by a prolonged infusion (8, 12, or 16 hours) was associated with a significantly greater increase in platelet function than placebo, as measured by multiple assays. Ticagrelor reversal occurred within 5 minutes after the initiation of PB2452 and was sustained for more than 20 hours (P<0.001 after Bonferroni adjustment across all time points for all assays). There was no evidence of a rebound in platelet activity after drug cessation. Adverse events related to the trial drug were limited mainly to issues involving the infusion site.
Conclusions
In healthy volunteers, the administration of PB2452, a specific reversal agent for ticagrelor, provided immediate and sustained reversal of the antiplatelet effects of ticagrelor, as measured by multiple assays.
Épargne transfusionnelle avec Fer IV+EPO+Vit B12+acide folique ?
Spahn et al., Lancet. 2019 Apr 26.
pii: S0140-6736(18)32555-8.
doi: 10.1016/S0140-6736(18)32555-8
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32555-8/fulltext
Background
Methods
Findings
Interpretation
Pour une prise en charge nutritionnelle individualisée avec diététicienne !
Prof Philipp Schuetz, et al. Lancet, 2019
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32776-4/fulltext
Background
Methods
Findings
Interpretation
Levetiracetam versus Phénytoïne dans l’état de mal épileptique ?
– Etude EcLiPSE
Lyttle et al., Lancet, 2019
DOI:https://doi.org/10.1016/S0140-6736(19)30724-X
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)30724-X/fulltext
– Etude ConSEPT
Dalziel et al., Lancet 2019
DOI:https://doi.org/10.1016/S0140-6736(19)30722-6
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)30722-6/fulltext
Traitement et Prévention du Delirium : haloperidol + lorazepam, le meilleur ?
Importance Although several pharmacological interventions for delirium have been investigated, their overall benefit and safety remain unclear.
Objective To evaluate evidence regarding pharmacological interventions for delirium treatment and prevention.
Data Sources PubMed, Embase, ProQuest, ScienceDirect, Cochrane Central, Web of Science, ClinicalKey, and ClinicalTrials.gov from inception to May 17, 2018.
Study Selection Randomized clinical trials (RCTs) examining pharmacological interventions for delirium treatment and prevention.
Data Extraction and Synthesis To extract data according to a predetermined list of interests, the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines were applied, and all meta-analytic procedures were conducted using a random-effects model.
Main Outcomes and Measures The primary outcomes were treatment response in patients with delirium and the incidence of delirium in patients at risk of delirium.
Results A total of 58 RCTs were included, in which 20 RCTs with 1435 participants (mean age, 63.5 years; 65.1% male) compared the outcomes of treatment and 38 RCTs with 8168 participants (mean age, 70.2 years; 53.4% male) examined the prevention of delirium. A network meta-analysis demonstrated that haloperidol plus lorazepam provided the best response rate for delirium treatment (odds ratio [OR], 28.13; 95% CI, 2.38-333.08) compared with placebo/control. For delirium prevention, the ramelteon, olanzapine, risperidone, and dexmedetomidine hydrochloride groups had significantly lower delirium occurrence rates than placebo/control (OR, 0.07; 95% CI, 0.01-0.66 for ramelteon; OR, 0.25; 95% CI, 0.09-0.69 for olanzapine; OR, 0.27; 95% CI, 0.07-0.99 for risperidone; and OR, 0.50; 95% CI, 0.31-0.80 for dexmedetomidine hydrochloride). None of the pharmacological treatments were significantly associated with a higher risk of all-cause mortality compared with placebo/control.
Conclusions and Relevance This network meta-analysis demonstrated that haloperidol plus lorazepam might be the best treatment and ramelteon the best preventive medicine for delirium. None of the pharmacological interventions for treatment or prophylaxis increased the all-cause mortality.
Refroidissement post-ACR par voie intra-nasale ?
Importance Therapeutic hypothermia may increase survival with good neurologic outcome after cardiac arrest. Trans-nasal evaporative cooling is a method used to induce cooling, primarily of the brain, during cardiopulmonary resuscitation (ie, intra-arrest).
Objective To determine whether prehospital trans-nasal evaporative intra-arrest cooling improves survival with good neurologic outcome compared with cooling initiated after hospital arrival.
Design, Setting, and Participants The PRINCESS trial was an investigator-initiated, randomized, clinical, international multicenter study with blinded assessment of the outcome, performed by emergency medical services in 7 European countries from July 2010 to January 2018, with final follow-up on April 29, 2018. In total, 677 patients with bystander-witnessed out-of-hospital cardiac arrest were enrolled.
Interventions Patients were randomly assigned to receive trans-nasal evaporative intra-arrest cooling (n = 343) or standard care (n = 334). Patients admitted to the hospital in both groups received systemic therapeutic hypothermia at 32°C to 34°C for 24 hours.
Main Outcomes and Measures The primary outcome was survival with good neurologic outcome, defined as Cerebral Performance Category (CPC) 1-2, at 90 days. Secondary outcomes were survival at 90 days and time to reach core body temperature less than 34°C.
Results Among the 677 randomized patients (median age, 65 years; 172 [25%] women), 671 completed the trial. Median time to core temperature less than 34°C was 105 minutes in the intervention group vs 182 minutes in the control group (P < .001). The number of patients with CPC 1-2 at 90 days was 56 of 337 (16.6%) in the intervention cooling group vs 45 of 334 (13.5%) in the control group (difference, 3.1% [95% CI, −2.3% to 8.5%]; relative risk [RR], 1.23 [95% CI, 0.86-1.72]; P = .25). In the intervention group, 60 of 337 patients (17.8%) were alive at 90 days vs 52 of 334 (15.6%) in the control group (difference, 2.2% [95% CI, −3.4% to 7.9%]; RR, 1.14 [95% CI, 0.81-1.57]; P = .44). Minor nosebleed was the most common device-related adverse event, reported in 45 of 337 patients (13%) in the intervention group. The adverse event rate within 7 days was similar between groups.
Conclusions and Relevance Among patients with out-of-hospital cardiac arrest, trans-nasal evaporative intra-arrest cooling compared with usual care did not result in a statistically significant improvement in survival with good neurologic outcome at 90 days.
Echec de l’hypothermie post-hémicraniectomie ?
Importance Moderate hypothermia in addition to early decompressive hemicraniectomy has been suggested to further reduce mortality and improve functional outcome in patients with malignant middle cerebral artery (MCA) stroke.
Objective To investigate whether moderate hypothermia vs standard treatment after early hemicraniectomy reduces mortality at day 14 in patients with malignant MCA stroke.
Design, Setting, and Participants This randomized clinical trial recruited patients from August 2011 through September 2015 at 6 German university hospitals with dedicated neurointensive care units. Of the patients treated with hemicraniectomy and assessed for eligibility, patients were randomly assigned to either standard care or moderate hypothermia. Data analysis was completed from December 2016 to June 2018.
Interventions Moderate hypothermia (temperature, 33.0 ± 1.0°C) was maintained for at least 72 hours immediately after hemicraniectomy.
Main Outcomes and Measures The primary outcome was mortality rate at day 14 compared with the Fisher exact test and expressed as odds ratio (ORs) with 95% CIs. Rates of patients with serious adverse events were estimated for the period of the first 14 days after hemicraniectomy and 12 months of follow-up. Secondary outcome measures included functional outcome at 12 months.
Results Of the 50 study participants, 24 were assigned to standard care and 26 to moderate hypothermia. Twenty-eight were male (56%); the mean (SD) patient age was 51.3 (6.6) years. Recruitment was suspended for safety concerns: 12 of 26 patients (46%) in the hypothermia group and 7 of 24 patients (29%) receiving standard care had at least 1 serious adverse event within 14 days (OR, 2.05 [95% CI, 0.56-8.00]; P = .26); after 12 months, rates of serious adverse events were 80% (n = 20 of 25) in the hypothermia group and 43% (n = 10 of 23) in the standard care group (hazard ratio, 2.54 [95% CI, 1.29-5.00]; P = .005). The mortality rate at day 14 was 19% (5 of 26 patients) in the hypothermia group and 13% (3 of 24 patients) in the group receiving standard care (OR, 1.65 [95% CI, 0.28-12.01]; P = .70). There was no significant difference regarding functional outcome after 12 months of follow-up.
Interpretation In patients with malignant MCA stroke, moderate hypothermia early after hemicraniectomy did not improve mortality and functional outcome compared with standard care, but may cause serious harm in this specific setting.
Hématome intracérébral profond : objectif de PAS entre 110-139 mmHg ?
Importance Hypertension is the strongest risk factor for spontaneous intracerebral hemorrhage (ICH) involving deep brain regions, but it appears to be unknown if intensive blood pressure reduction in the acute care setting decreases hematoma expansion or improves outcomes in patients with deep ICH.
Objective To determine whether intensive blood pressure reduction is associated with decreased risk of hematoma expansion and changes in 90-day modified Rankin Scale scores and if these associations are modified by the specific deep-brain nuclei involved.
Design, Setting, and Participants This study is an exploratory analysis of the Antihypertensive Treatment of Acute Cerebral Hemorrhage–2 international, multicenter randomized clinical trial, which was conducted from May 2011 to September 2015, enrolled eligible patients with primary ICH, and followed up with them for 90 days. Patients who had ICH and complete neuroimaging data were included in the analysis. Data analysis was completed from July 2018 to December 2018.
Exposures Participants were randomized to either intensive treatment (with a systolic blood pressure target of 110-139 mm Hg) or standard treatment (with a systolic blood pressure target of 140-179 mm Hg).
Main Outcomes and Measures The main outcome was hematoma expansion, defined as an increase greater than 33% in hematoma volume between baseline and 24 hours. Functional outcome was evaluated 90 days after the ICH via the modified Rankin Scale.
Results Of 1000 trial participants, 870 (87.0%) had deep ICH, of whom 780 (89.7%) had complete neuroimaging data (of 336 thalamic and 444 basal ganglia hemorrhages). The baseline characteristics of the intensive and standard treatment groups remained balanced in this subgroup of the original study. Intensive treatment was associated with a decreased risk of hematoma expansion in univariable analysis (odds ratio [OR], 0.62 [95% CI, 0.43-0.87]; P = .006) and multivariable analysis (OR, 0.61 [95% CI, 0.42-0.88]; P = .009). This association was modified by the specific deep location of the ICH (OR, 0.44 [95% CI, 0.22-0.96]; interaction P = .02), with stratified analyses showing a reduction in risk of hematoma expansion with intensive vs standard treatment among basal ganglia ICH (OR, 0.44 [95% CI, 0.27-0.72]; P = .001) but not thalamic ICH (OR, 0.91 [95% CI, 0.51-0.64]; P = .76). Intensive treatment was not associated with an improvement in the modified Rankin Scale score distribution.
Conclusions and Relevance Compared with standard treatment, intensive blood pressure treatment was associated with reduced hematoma expansion in deep ICH, specifically among basal ganglia hemorrhages.
Objectif de PAM adapté au seuil d’autorégulation cérébrale en chirurgie cardiaque ?
Importance Delirium occurs in up to 52% of patients after cardiac surgery and may result from changes in cerebral perfusion. Using intraoperative cerebral autoregulation monitoring to individualize and optimize cerebral perfusion may be a useful strategy to reduce the incidence of delirium after cardiac surgery.
Objective To determine whether targeting mean arterial pressure during cardiopulmonary bypass (CPB) using cerebral autoregulation monitoring reduces the incidence of delirium compared with usual care.
Design, Setting, and Participants This randomized clinical trial nested within a larger trial enrolled patients older than 55 years who underwent nonemergency cardiac surgery at a single US academic medical center between October 11, 2012, and May 10, 2016, and had a high risk for neurologic complications. Patients, physicians, and outcome assessors were masked to the assigned intervention. A total of 2764 patients were screened, and 199 were eligible for analysis in this study.
Intervention In the intervention group, the patient’s lower limit of cerebral autoregulation was identified during surgery before CPB. On CPB, the patient’s mean arterial pressure was targeted to be greater than that patient’s lower limit of autoregulation. In the control group, mean arterial pressure targets were determined according to institutional practice.
Main Outcomes and Measures The main outcome was any incidence of delirium on postoperative days 1 through 4, as adjudicated by a consensus expert panel.
Results Among the 199 participants in this study, mean (SD) age was 70.3 (7.5) years and 150 (75.4%) were male. One hundred sixty-two (81.4%) were white, 26 (13.1%) were black, and 11 (5.5%) were of other race. Of 103 patients randomized to usual care, 94 were analyzed, and of 102 patients randomized to the intervention 105 were analyzed. Excluding 5 patients with coma, delirium occurred in 48 of the 91 patients (53%) in the usual care group compared with 39 of the 103 patients (38%) in the intervention group (P = .04). The odds of delirium were reduced by 45% in patients randomized to the autoregulation group (odds ratio, 0.55; 95% CI, 0.31-0.97; P = .04).
Conclusions and Relevance The results of this study suggest that optimizing mean arterial pressure to be greater than the individual patient’s lower limit of cerebral autoregulation during CPB may reduce the incidence of delirium after cardiac surgery, but further study is needed.
Machine Learning : 4 phénotypes identifiés du sepsis ?
https://jamanetwork.com/journals/jama/article-abstract/2733996
Importance Sepsis is a heterogeneous syndrome. Identification of distinct clinical phenotypes may allow more precise therapy and improve care.
Objective To derive sepsis phenotypes from clinical data, determine their reproducibility and correlation with host-response biomarkers and clinical outcomes, and assess the potential causal relationship with results from randomized clinical trials (RCTs).
Design, Settings, and Participants Retrospective analysis of data sets using statistical, machine learning, and simulation tools. Phenotypes were derived among 20 189 total patients (16 552 unique patients) who met Sepsis-3 criteria within 6 hours of hospital presentation at 12 Pennsylvania hospitals (2010-2012) using consensus k means clustering applied to 29 variables. Reproducibility and correlation with biological parameters and clinical outcomes were assessed in a second database (2013-2014; n = 43 086 total patients and n = 31 160 unique patients), in a prospective cohort study of sepsis due to pneumonia (n = 583), and in 3 sepsis RCTs (n = 4737).
Exposures All clinical and laboratory variables in the electronic health record.
Main Outcomes and Measures Derived phenotype (α, β, γ, and δ) frequency, host-response biomarkers, 28-day and 365-day mortality, and RCT simulation outputs.
Results The derivation cohort included 20 189 patients with sepsis (mean age, 64 [SD, 17] years; 10 022 [50%] male; mean maximum 24-hour Sequential Organ Failure Assessment [SOFA] score, 3.9 [SD, 2.4]). The validation cohort included 43 086 patients (mean age, 67 [SD, 17] years; 21 993 [51%] male; mean maximum 24-hour SOFA score, 3.6 [SD, 2.0]). Of the 4 derived phenotypes, the α phenotype was the most common (n = 6625; 33%) and included patients with the lowest administration of a vasopressor; in the β phenotype (n = 5512; 27%), patients were older and had more chronic illness and renal dysfunction; in the γ phenotype (n = 5385; 27%), patients had more inflammation and pulmonary dysfunction; and in the δ phenotype (n = 2667; 13%), patients had more liver dysfunction and septic shock. Phenotype distributions were similar in the validation cohort. There were consistent differences in biomarker patterns by phenotype. In the derivation cohort, cumulative 28-day mortality was 287 deaths of 5691 unique patients (5%) for the α phenotype; 561 of 4420 (13%) for the β phenotype; 1031 of 4318 (24%) for the γ phenotype; and 897 of 2223 (40%) for the δ phenotype. Across all cohorts and trials, 28-day and 365-day mortality were highest among the δ phenotype vs the other 3 phenotypes (P < .001). In simulation models, the proportion of RCTs reporting benefit, harm, or no effect changed considerably (eg, varying the phenotype frequencies within an RCT of early goal-directed therapy changed the results from >33% chance of benefit to >60% chance of harm).
Conclusions and Relevance In this retrospective analysis of data sets from patients with sepsis, 4 clinical phenotypes were identified that correlated with host-response patterns and clinical outcomes, and simulations suggested these phenotypes may help in understanding heterogeneity of treatment effects. Further research is needed to determine the utility of these phenotypes in clinical care and for informing trial design and interpretation.
Lovenox 4000UI SC pour tous : Alerte Dogme Faux ?
https://jamanetwork.com/journals/jamasurgery/article-abstract/2734658
Importance Between 4% and 12% of patients undergoing colorectal surgery and receiving enoxaparin, 40 mg per day, have a postoperative VTE event. An improved understanding of why “breakthrough” VTE events occur despite guideline-compliant prophylaxis is an important patient safety question.
Objective To determine the proportion of patients undergoing colorectal surgery who received adequate anticoagulation based on peak anti–factor Xa (aFXa) levels while receiving enoxaparin at 40 mg per day.
Design, Setting, and Participants This prospective, nonrandomized clinical trial was conducted between February 2017 and July 2018 with 90-day follow-up at a quaternary academic medical center in the Intermountain West and included patients undergoing colorectal surgery who had surgery after receiving general anesthesia, were admitted for at least 3 days, and received enoxaparin, 40 mg once daily.
Interventions All patients had aFXa levels measured after receiving enoxaparin 40mg per day. Patients whose aFXa level was out of range entered the trial’s interventional arm where real-time enoxaparin dose adjustment and repeated aFXa measurement were performed.
Main Outcomes and Measures Primary outcome: in-range peak aFXa levels (goal range, 0.3-0.5 IU/mL) with enoxaparin, 40 mg per day. Secondary outcomes: (1) in-range trough aFXa levels (goal range, 0.1-0.2 IU/mL) and (2) the proportion of patients with in-range peak aFXa levels from enoxaparin, 40 mg once daily, vs the real-time enoxaparin dose adjustment protocol.
Results Over 16 months, 116 patients undergoing colorectal surgery (65 women [56.0%]; 99 white individuals [85.3%], 13 Hispanic or Latino individuals [11.2%], and 4 Pacific Islander individuals [3.5%]; mean [range] age, 52.1 [18-85] years) were enrolled. Among 106 patients (91.4%) whose peak aFXa level was appropriately drawn, 72 (67.9%) received inadequate anticoagulation (aFXa < 0.3 IU/mL) with enoxaparin, 40 mg per day. Weight and peak aFXa levels were inversely correlated (r2 = 0.38). Forty-seven patients (77%) had a trough aFXa level that was not detectable (ie, most patients had no detectable level of anticoagulation for at least 12 hours per day). Real-time enoxaparin dose adjustment was effective. Patients were significantly more likely to achieve an in-range peak aFXa with real-time dose adjustment as opposed to fixed dosing alone (85.4% vs 29.2%, P < .001).
Conclusions and Relevance This study supports the finding that most patients undergoing colorectal surgery receive inadequate prophylaxis from enoxaparin, 40 mg once daily. These findings may explain the high rate of “breakthrough” VTE observed in many clinical trials.
Epidémio des états de mort encéphalique en Réa péd
https://jamanetwork.com/journals/jamapediatrics/fullarticle/
Les vieux meurent plus après leur sortie de réa mais les jeunes aussi à plus long terme ?
Importance An aging population is increasing the need for intensive care unit (ICU) beds. The benefit of ICU admission for elderly patients remains a subject of debate; however, long-term outcomes across all adult age strata are unknown.
Objective To describe short-term and long-term mortality (up to 3 years after discharge) across age strata in adult patients admitted to French ICUs.
Design, Setting, and Participants Using data extracted from the French national health system database, this cohort study determined in-hospital mortality and mortality at 3 months and 3 years after discharge of adult patients (older than 18 years) admitted to French ICUs from January 1 to December 31, 2013, focusing on age strata. The dates of analysis were November 2017 to December 2018.
Exposure Intensive care unit admission.
Main Outcomes and Measures In-hospital mortality and mortality at 3 months and 3 years after hospital discharge.
Results The study included 133 966 patients (median age, 65 years [interquartile range, 53-76 years); 59.9% male). Total in-hospital mortality was 19.0%, and 3-year mortality was 39.7%. For the 108 539 patients discharged alive from the hospital, 6.8% died by 3 months, and 25.8% died by 3 years after hospital discharge. After adjustment for sex, comorbidities, reason for hospitalization, and organ support (invasive ventilation, noninvasive ventilation, vasopressors, inotropes, fluid resuscitation, blood products administration, cardiopulmonary resuscitation, renal replacement therapy, and mechanical circulatory support), risk of mortality increased progressively across all age strata but with a sharp increase in those 80 years and older. In-hospital and 3-year postdischarge mortality rates, respectively, were 30.5% and 44.9% in patients 80 years and older compared with 16.5% and 22.5% in those younger than 80 years. Total 3-year mortality was 61.4% among patients 80 years and older vs 35.1% in those younger than 80. After age and sex standardization, excess mortality was highest among young patients during their first year after hospital discharge and persisted into the second and third years. In contrast, the mortality risk was close to the general population risk among elderly patients (≥80 years). Age and reason for hospitalization were strongly associated with long-term mortality (9-, 13-, and 20-fold increase in the risk of death 3 years after ICU discharge in patients aged 80-84, 85-89, and ≥90 years, respectively, compared with patients aged <35 years), while organ support use during ICU showed a weaker association (all organ support had 1.3-fold or lower increase in the risk of death).
Conclusions and Relevance Results of this study suggest that aging was associated with an increased risk of mortality in the 3 years after hospital discharge that included an ICU admission, with a sharp increase in those 80 years and older. However, compared with the general population matched by age and sex, excess long-term mortality was high in young surviving patients but not in elderly patients.
Recommendations péri-opératoire de chirurgie cardiaque
https://jamanetwork.com/journals/jamasurgery/fullarticle/2732511
Effet antibiotique du Ticagrelor ?
Les complications post-opératoires, source de mal-être de nos amis chirurgiens ?
https://jamanetwork.com/journals/jamasurgery/article-abstract/2729453
Méta-analyse sur la Vasopressine dans le choc septique
https://link.springer.com/article/10.1007%2Fs00134-019-05620-2
Remplissage restrictif (<60mL/kg) dans le choc septique ?
Boughton CK, et al. Lancet Diabetes Endocrinol. 2019 May;7(5):368-377.
doi: 10.1016/S2213-8587(19)30061-0.
https://insights.ovid.com/pubmed?pmid=30985449
OBJECTIVES:
It is unclear if a low- or high-volume IV fluid resuscitation strategy is better for patients with severe sepsis and septic shock.
DESIGN:
Prospective randomized controlled trial.
SETTING:
Two adult acute care hospitals within a single academic system.
PATIENTS:
Patients with severe sepsis and septic shock admitted from the emergency department to the ICU from November 2016 to February 2018.
INTERVENTIONS:
Patients were randomly assigned to a restrictive IV fluid resuscitation strategy (≤ 60 mL/kg of IV fluid) or usual care for the first 72 hours of care.
MEASUREMENTS AND MAIN RESULTS:
We enrolled 109 patients, of whom 55 were assigned to the restrictive resuscitation group and 54 to the usual care group. The restrictive group received significantly less resuscitative IV fluid than the usual care group (47.1 vs 61.1 mL/kg; p = 0.01) over 72 hours. By 30 days, there were 12 deaths (21.8%) in the restrictive group and 12 deaths (22.2%) in the usual care group (odds ratio, 1.02; 95% CI, 0.41-2.53). There were no differences between groups in the rate of new organ failure, hospital or ICU length of stay, or serious adverse events.
CONCLUSIONS:
This pilot study demonstrates that a restrictive resuscitation strategy can successfully reduce the amount of IV fluid administered to patients with severe sepsis and septic shock compared with usual care. Although limited by the sample size, we observed no increase in mortality, organ failure, or adverse events. These findings further support that a restrictive IV fluid strategy should be explored in a larger multicenter trial.
CGR frais versus plus vieux : NS
Irving et al., Critical Care Medicine. Publish Ahead of Print():, APR 2019
DOI: 10.1097/CCM.0000000000003781
https://insights.ovid.com/crossref?an=00003246-900000000-95965
Objectives:
Trials comparing the effects of transfusing RBC units of different storage durations have considered mortality or morbidity as outcomes. We perform the first economic evaluation alongside a full age of blood clinical trial with a large population assessing the impact of RBC storage duration on quality-of-life and costs in critically ill adults.
Design:
Quality-of-life was measured at 6 months post randomization using the EuroQol 5-dimension 3-level instrument. The economic evaluation considers quality-adjusted life year and cost implications from randomization to 6 months. A generalized linear model was used to estimate incremental costs (2016 U.S. dollars) and quality-adjusted life years, respectively while adjusting for baseline characteristics.
Setting:
Fifty-nine ICUs in five countries.Patients:Adults with an anticipated ICU stay of at least 24 hours when the decision had been made to transfuse at least one RBC unit.
Interventions:
Patients were randomized to receive either the freshest or oldest available compatible RBC units (standard practice) in the hospital transfusion service.
Measurements and Main Results:
EuroQol 5-dimension 3-level utility scores were similar at 6 months—0.65 in the short-term and 0.63 in the long-term storage group (difference, 0.02; 95% CI, –0.00 to 0.04; p = 0.10). There were no significant differences in resource use between the two groups apart from 3.0 fewer hospital readmission days (95% CI, –5.3 to –0.8; p = 0.01) during follow-up in the short-term storage group. There were no significant differences in adjusted total costs or quality-adjusted life years between the short- and long-term storage groups (incremental costs, –$2,358; 95% CI, –$5,586 to $711) and incremental quality-adjusted life years: 0.003 quality-adjusted life years (95% CI, –0.003 to 0.008).
Conclusions:
Without considering the additional supply cost of implementing a freshest available RBC strategy for critical care patients, there is no evidence to suggest that the policy improves quality-of-life or reduces other costs compared with standard transfusion practice.
Pupillométrie comme monitorage de la PIC chez les traumatisés crâniens
Fritz-Patrick Jahns, et al.
https://ccforum.biomedcentral.com/articles/10.1186/s13054-019-2436-3
Background
Elevated intracranial pressure (ICP) is frequent after traumatic brain injury (TBI) and may cause abnormal pupillary reactivity, which in turn is associated with a worse prognosis. Using automated infrared pupillometry, we examined the relationship between the Neurological Pupil index (NPi) and invasive ICP in patients with severe TBI.
Methods
This was an observational cohort of consecutive subjects with severe TBI (Glasgow Coma Scale [GCS] < 9 with abnormal lesions on head CT) who underwent parenchymal ICP monitoring and repeated NPi assessment with the NPi-200® pupillometer. We examined NPi trends over time (four consecutive measurements over intervals of 6 h) prior to sustained elevated ICP > 20 mmHg. We further analyzed the relationship of cumulative abnormal NPi burden (%NPi values < 3 during total ICP monitoring time) with intracranial hypertension (ICHT)—categorized as refractory (ICHT-r; requiring surgical decompression) vs. non-refractory (ICHT-nr; responsive to medical therapy)—and with the 6-month Glasgow Outcome Score (GOS).
Results
A total of 54 patients were studied (mean age 54 ± 21 years, 74% with focal injuries on CT), of whom 32 (59%) had ICHT. Among subjects with ICHT, episodes of sustained elevated ICP (n = 43, 172 matched ICP-NPi samples; baseline ICP [T− 6 h] 14 ± 5 mmHg vs. ICPmax [T0 h] 30 ± 9 mmHg) were associated with a concomitant decrease of the NPi (baseline 4.2 ± 0.5 vs. 2.8 ± 1.6, p < 0.0001 ANOVA for repeated measures). Abnormal NPi values were more frequent in patients with ICHT-r (n = 17; 38 [3–96]% of monitored time vs. 1 [0–9]% in patients with ICHT-nr [n = 15] and 0.5 [0–10]% in those without ICHT [n = 22]; p = 0.007) and were associated with an unfavorable 6-month outcome (15 [1–80]% in GOS 1–3 vs. 0 [0–7]% in GOS 4–5 patients; p = 0.002).
Conclusions
In a selected cohort of severe TBI patients with abnormal head CT lesions and predominantly focal cerebral injury, elevated ICP episodes correlated with a concomitant decrease of NPi. Sustained abnormal NPi was in turn associated with a more complicated ICP course and worse outcome.
Grippe et co-infections chez les patients immunodéprimés en réanimation
Ignacio Martin-Loeches, et al.
https://ccforum.biomedcentral.com/articles/10.1186/s13054-019-2425-6
Background
It is unclear whether influenza infection and associated co-infection are associated with patient-important outcomes in critically ill immunocompromised patients with acute respiratory failure.
Methods
Preplanned secondary analysis of EFRAIM, a prospective cohort study of 68 hospitals in 16 countries. We included 1611 patients aged 18 years or older with non-AIDS-related immunocompromise, who were admitted to the ICU with acute hypoxemic respiratory failure. The main exposure of interest was influenza infection status. The primary outcome of interest was all-cause hospital mortality, and secondary outcomes ICU length of stay (LOS) and 90-day mortality.
Results
Influenza infection status was categorized into four groups: patients with influenza alone (n = 95, 5.8%), patients with influenza plus pulmonary co-infection (n = 58, 3.6%), patients with non-influenza pulmonary infection (n = 820, 50.9%), and patients without pulmonary infection (n = 638, 39.6%). Influenza infection status was associated with a requirement for intubation and with LOS in ICU (P < 0.001). Patients with influenza plus co-infection had the highest rates of intubation and longest ICU LOS. On crude analysis, influenza infection status was associated with ICU mortality (P < 0.001) but not hospital mortality (P = 0.09). Patients with influenza plus co-infection and patients with non-influenza infection alone had similar ICU mortality (41% and 37% respectively) that was higher than patients with influenza alone or those without infection (33% and 26% respectively). A propensity score-matched analysis did not show a difference in hospital mortality attributable to influenza infection (OR = 1.01, 95%CI 0.90–1.13, P = 0.85). Age, severity scores, ARDS, and performance status were all associated with ICU, hospital, and 90-day mortality.
Conclusions
Category of infectious etiology of respiratory failure (influenza, non-influenza, influenza plus co-infection, and non-infectious) was associated with ICU but not hospital mortality. In a propensity score-matched analysis, influenza infection was not associated with the primary outcome of hospital mortality. Overall, influenza infection alone may not be an independent risk factor for hospital mortality in immunosuppressed patients.
Fixateur de sonde d’IOT > fixation avec du scotch ?
Janna S. Landsperger, et al., Critical Care 2019 23:161
https://ccforum.biomedcentral.com/articles/10.1186/s13054-019-2440-7
Background
The optimal securement method of endotracheal tubes is unknown but should prevent dislodgement while minimizing complications. The use of an endotracheal tube fastener might reduce complications among critically ill adults undergoing endotracheal intubation.
Methods
In this pragmatic, single-center, randomized trial, critically ill adults admitted to the medical intensive care unit (MICU) and expected to require invasive mechanical ventilation for greater than 24 h were randomized to adhesive tape or endotracheal tube fastener at the time of intubation. The primary endpoint was a composite of any of the following: presence of lip ulcer, endotracheal tube dislodgement (defined as moving at least 2 cm), ventilator-associated pneumonia, or facial skin tears anytime between randomization and the earlier of death or 48 h after extubation. Secondary endpoints included duration of mechanical ventilation and ICU and in-hospital mortality.
Results
Of 500 patients randomized over a 12-month period, 162 had a duration of mechanical ventilation less than 24 h and 40 had missing outcome data, leaving 153 evaluable patients randomized to tube fastener and 145 evaluable patients randomized to adhesive tape. Baseline characteristics were similar between the groups. The primary endpoint occurred 13 times in 12 (7.8%) patients in the tube fastener group and 30 times in 25 (17.2%) patients in the adhesive tape group (p = 0.014) for an overall incidence of 22.0 versus 52.6 per 1000 ventilator days, respectively (p = 0.020). Lip ulcers occurred in 4 (2.6%) versus 11 (7.3%) patients, or an incidence rate of 6.5 versus 19.5 per 1000 patient ventilator days (p = 0.053) in the fastener and tape groups, respectively. The endotracheal tube was dislodged 7 times in 6 (3.9%) patients in the tube fastener group and 16 times in 15 (10.3%) patients in the tape group (p = 0.03), reflecting incidences of 11.9 and 28.1 per 1000 ventilator days, respectively. Facial skin tears were similar between the groups. Mechanical ventilation duration and ICU and hospital mortality did not differ.
Conclusion
The use of the endotracheal tube fastener to secure the endotracheal tubes reduces the rate of a composite outcome that included lip ulcers, facial skin tears, or endotracheal tube dislodgement compared to adhesive tape.
Méta-analyse : NAD+Dobu, la meilleure combinaison pour le choc septique ?
Lu Cheng, et al., Critical Care 2019
https://ccforum.biomedcentral.com/articles/10.1186/s13054-019-2427-4
Echec de la Dexaméthasone a prolongé la durée d’un bloc ?
https://bjanaesthesia.org/article/S0007-0912(19)30016-9/fulltext
Background
The efficacy of dexamethasone in extending the duration of local anaesthetic block is uncertain. In a randomised controlled triple blind crossover study in volunteers, we tested the hypothesis that neither i.v. nor perineurally administered dexamethasone prolongs the sensory block achieved with ropivacaine.
Methods
Ultrasound-guided ulnar nerve blocks (ropivacaine 0.75% wt/vol, 3 ml, with saline 1 ml with or without dexamethasone 4 mg) were performed on three occasions in 24 male volunteers along with an i.v. injection of saline 1 ml with or without dexamethasone 4 mg. The combinations of saline and dexamethasone were as follows: control group, perineural and i.v. saline; perineural group, perineural dexamethasone and i.v. saline; i.v. group, perineural saline and i.v. dexamethasone. Sensory block was measured using a VAS in response to pinprick testing. The duration of sensory block was the primary outcome and time to onset of sensory block the secondary outcome.
Results
All 24 subjects completed the trial. The median [inter-quartile range (IQR)] duration of sensory block was 6.87 (5.85–7.62) h in the control group, 7.37 (5.78–7.93) h in the perineural group and 7.37 (6.10–7.97) h in the i.v. group (P=0.61). There was also no significant difference in block onset time between the three groups.
Conclusion
Dexamethasone 4 mg has no clinically relevant effect on the duration of sensory block provided by ropivacaine applied to the ulnar nerve.
La sédation par Propofol sans morphiniques, pas associé à plus de delirium ?
Abstract
Background
The Strategy to Reduce the Incidence of Postoperative Delirium in the Elderly trial tested the hypothesis that limiting sedation during spinal anaesthesia decreases in-hospital postoperative delirium after hip fracture repair. This manuscript reports the secondary outcomes of this trial, including mortality and function.
Methods
Two hundred patients (≥65 yr) undergoing hip fracture repair with spinal anaesthesia were randomised to heavier [modified Observer’s Assessment of Alertness/Sedation score (OAA/S) 0–2] or lighter (OAA/S 3–5) sedation, and were assessed for postoperative delirium. Secondary outcomes included mortality and return to pre-fracture ambulation level at 1 yr. Kaplan–Meier analysis, multivariable Cox proportional hazard model, and logistic regression were used to evaluate intervention effects on mortality and odds of ambulation return.
Results
One-year mortality was 14% in both groups (log rank P=0.96). Independent risk factors for 1-yr mortality included: Charlson comorbidity index [hazard ratio (HR)=1.23, 95% confidence interval (CI), 1.02–1.49; P=0.03], instrumental activities of daily living [HR=0.74, 95% CI, 0.60–0.91; P=0.005], BMI [HR=0.91, 95% CI 0.84–0.998; P=0.04], and delirium severity [HR=1.20, 95% CI, 1.03–1.41; P=0.02]. Ambulation returned to pre-fracture levels, worsened, or was not obtained in 64%, 30%, and 6% of 1 yr survivors, respectively. Lighter sedation did not improve odds of ambulation return at 1 yr [odds ratio (OR)=0.76, 95% CI, 0.24–2.4; P=0.63]. Independent risk factors for ambulation return included Charlson comorbidity index [OR=0.71, 95% CI, 0.53–0.97; P=0.03] and delirium [OR=0.32, 95% CI, 0.10–0.97; P=0.04].
Conclusions
This study found that in elderly patients having hip fracture surgery with spinal anaesthesia supplemented with propofol sedation, heavier intraoperative sedation was not associated with significant differences in mortality or return to pre-fracture ambulation up to 1 yr after surgery.
Bloc triangle fémoral versus canal des adducteurs pour les PTG : NS ??
https://bjanaesthesia.org/article/S0007-0912(19)30225-9/fulltext
Background
Adductor canal (AC) catheters are being used to provide continuous postoperative analgesia after total knee arthroplasty (TKA) surgery. There are anatomical arguments that most AC catheters are being inserted into the femoral triangle (FT) compartment of the thigh rather than the AC compartment. The clinical relevance of this is unknown with respect to motor weakness, quality of analgesia, and opioid consumption. We hypothesised that AC catheters provide superior functional mobilisation on postoperative Day 1 after TKA as measured using the Timed Up and Go (TUG) test.
Methods
In this multinational, multicentre, double-blinded RCT, catheters were inserted under ultrasound guidance into the anatomical AC and FT compartments. The standardised protocol included spinal anaesthesia without intrathecal morphine, fixed catheter infusion rates, and oral analgesia.
Results
Of 151 subjects recruited, 75 were in the AC group and 76 in the FT group. There was no statistically significant difference in TUG on postoperative Day 1 between AC (38 [29–55] s) and FT subjects (44 [32–64] s) (median [inter-quartile range]); P=0.11). There was no difference in TUG Day 2, AC (38 [27–53] s) vs FT (42 [31–59] s); P=0.66. There were no statistically significant differences for secondary endpoints of pain level, effectiveness of pain relief, interference of functional activities and interpersonal relationships by pain, and opioid consumption between groups.
Conclusions
There were no differences in immediate postoperative functional mobility, analgesia, and opioid consumption provided by catheters inserted into the AC vs FT locations for TKA surgery.
Utilisation de la driving pressure en chirurgie thoracique : moins de complications
Background: Recently, several retrospective studies have suggested that pulmonary complication is related with driving pressure more than any other ventilatory parameter. Thus, the authors compared driving pressure–guided ventilation with conventional protective ventilation in thoracic surgery, where lung protection is of the utmost importance. The authors hypothesized that driving pressure–guided ventilation decreases postoperative pulmonary complications more than conventional protective ventilation.
Methods: In this double-blind, randomized, controlled study, 292 patients scheduled for elective thoracic surgery were included in the analysis. The protective ventilation group (n = 147) received conventional protective ventilation during one-lung ventilation: tidal volume 6 ml/kg of ideal body weight, positive end-expiratory pressure (PEEP) 5 cm H2O, and recruitment maneuver. The driving pressure group (n = 145) received the same tidal volume and recruitment, but with individualized PEEP which produces the lowest driving pressure (plateau pressure–PEEP) during one-lung ventilation. The primary outcome was postoperative pulmonary complications based on the Melbourne Group Scale (at least 4) until postoperative day 3.
Results: Melbourne Group Scale of at least 4 occurred in 8 of 145 patients (5.5%) in the driving pressure group, as compared with 18 of 147 (12.2%) in the protective ventilation group (P = 0.047, odds ratio 0.42; 95% CI, 0.18 to 0.99). The number of patients who developed pneumonia or acute respiratory distress syndrome was less in the driving pressure group than in the protective ventilation group (10/145 [6.9%] vs. 22/147 [15.0%], P = 0.028, odds ratio 0.42; 95% CI, 0.19 to 0.92).
Conclusions: Application of driving pressure–guided ventilation during one-lung ventilation was associated with a lower incidence of postoperative pulmonary complications compared with conventional protective ventilation in thoracic surgery.
Le ppt en cadeau : Biblio – driving pressure chir tho
Précharge dépendance = altération micro-circulatoire ?
Background: Dynamic indices, such as pulse pressure variation, detect preload dependence and are used to predict fluid responsiveness. The behavior of sublingual microcirculation during preload dependence is unknown during major abdominal surgery. The purpose of this study was to test the hypothesis that during abdominal surgery, microvascular perfusion is impaired during preload dependence and recovers after fluid administration.
Methods: This prospective observational study included patients having major abdominal surgery. Pulse pressure variation was used to identify preload dependence. A fluid challenge was performed when pulse pressure variation was greater than 13%. Macrocirculation variables (mean arterial pressure, heart rate, stroke volume index, and pulse pressure variation) and sublingual microcirculation variables (perfused vessel density, microvascular flow index, proportion of perfused vessels, and flow heterogeneity index) were recorded every 10 min.
Results: In 17 patients, who contributed 32 preload dependence episodes, the occurrence of preload dependence during major abdominal surgery was associated with a decrease in mean arterial pressure (72 ± 9 vs. 83 ± 15 mmHg [mean ± SD]; P = 0.016) and stroke volume index (36 ± 8 vs. 43 ± 8 ml/m2; P < 0.001) with a concomitant decrease in microvascular flow index (median [interquartile range], 2.33 [1.81, 2.75] vs. 2.84 [2.56, 2.88]; P = 0.009) and perfused vessel density (14.9 [12.0, 16.4] vs. 16.1 mm/mm2 [14.7, 21.4], P = 0.009), while heterogeneity index was increased from 0.2 (0.2, 0.4) to 0.5 (0.4, 0.7; P = 0.001). After fluid challenge, all microvascular parameters and the stroke volume index improved, while mean arterial pressure and heart rate remained unchanged.
Conclusions: Preload dependence was associated with reduced sublingual microcirculation during major abdominal surgery. Fluid administration successfully restored microvascular perfusion.
HEA versus Ringer Lactate : NS
Background: Crystalloid solutions leave the circulation quickly, whereas colloids remain for hours, thus promoting hemodynamic stability. However, colloids are expensive and promote renal toxicity in critical care patients. This study tested the hypothesis that goal-directed colloid administration during elective abdominal surgery decreases 30-day major complications more than goal-directed crystalloid administration.
Methods: In this parallel-arm double-blinded multicenter randomized trial, adults having moderate- to high-risk open and laparoscopically assisted abdominal surgery with general anesthesia were randomly assigned to Doppler-guided intraoperative volume replacement with 6% hydroxyethyl starch 130/0.4 (n = 523) or lactated Ringer’s solution (n = 534). The primary outcome was a composite of serious postoperative cardiac, pulmonary, infectious, gastrointestinal, renal, and coagulation complications that were assessed with a generalized estimating equation multivariate model. The primary safety outcome was a change in serum creatinine concentration up to 6 months postoperatively, compared to baseline concentrations.
Results: A total of 1,057 patients were included in the analysis. Patients assigned to crystalloid received a median [quartile 1, quartile 3] amount of 3.2 l [2.3, 4.4] of crystalloid, and patients assigned to colloid received 1.0 l [0.5, 1.5] of colloid and 1.8 l [1.2, 2.4] of crystalloid. The estimated intention-to-treat common effect relative risk for the primary composite was 0.90 for colloids versus crystalloids (95% CI: 0.65 to 1.23, P = 0.51), and 18% (91 of 523) of colloid patients and 20% (103 of 534) of crystalloid patients incurred at least one component of the primary outcome composite. There was no evidence of renal toxicity at any time.
Conclusions: Doppler-guided intraoperative hydroxyethyl starch administration did not significantly reduce a composite of serious complications. However, there was also no indication of renal or other toxicity.
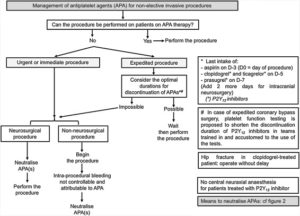
Recommendations du GIHP sur la Gestion des anti-plaquettaires
https://doi.org/10.1016/j.accpm.2018.10.004
https://www.sciencedirect.com/science/article/pii/S2352556818304156?via%3Dihub
Méta-analyse : Pas d’intérêt à une antibioprophylaxie post-ACR
https://www.resuscitationjournal.com/article/S0300-9572(19)30164-9/fulltext
Echec de l’ECCO2R pour une ventilation protectrice ?
https://link.springer.com/article/10.1007%2Fs10047-018-1068-8





 28 mai 2019
28 mai 2019










 Étiquettes :
Étiquettes :