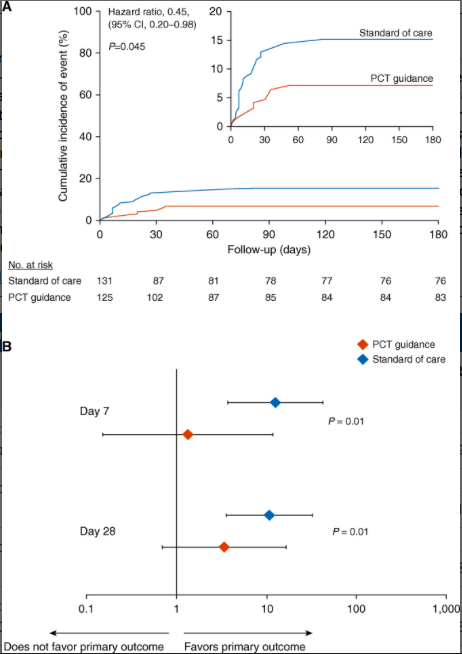
Study primary outcome and the effect of fecal colonization in the intention-to-treat population. (A) Kaplan-Meier curve for primary outcome. The study primary outcome was the rate of infection-associated adverse events for patients allocated to the PCT-guidance group compared with the standard of care after 180 days. Infection-associated adverse events were a composite outcome comprising the advent of any of the following: new case of Clostridioides difficile infection; new case of infection by multidrug-resistant organisms (MDROs); and death due to baseline infection by MDROs or C. difficile. The inset shows the same data on an enlarged y-axis. (B) Odds ratios of reaching or not the primary outcome in dependence of presence or absence of fecal colonization by C. difficile or MDROs by Days 7 and 28. P values of the interaction effect of the arm of treatment by colonization on primary outcome are provided. CI = confidence interval; PCT = procalcitonin.




